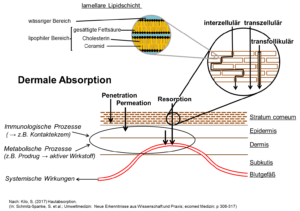
Skin resorption
The body surface of an adult man covers about 2 m2, somewhat less than that of an adult woman. This surface is covered with skin. Specially differentiated cells and components of the skin are responsible for, among other tasks, homeostasis and the perception of pain, temperature, and pressure. Skin forms a barrier that separates us from our environment and protects us from water loss. A substance must first penetrate this skin barrier to reach the blood stream and to be absorbed into the system. The most external skin layer, the stratum corneum, forms the primary part of this barrier. The stratum corneum is composed of 10–20 layers of callous, seedless keratinocytes or corneocytes which are embedded to form a lamellar lipid layer in a “brick-and-mortar” model (Elias 1983). The corneocytes are stabilised against displacement by punctiform, desmosomal compounds (“rivets”) and interlocked, hook-shaped surface structures. After the formation of a basal cell layer, keratinocytes travel through the epidermis, become differentiated from corneocytes, and are finally cast off (dandruff).
Chemicals generally pass through the stratum corneum by passive diffusion, whereby speed and amount as well as the physicochemical characteristics of the chemicals and the skin itself (i.e. diffusion area and distance) are factors. The stratum corneum may be crossed by substances intercellularly (see Figure), which means that substances diffuse into the lipid layer along the corneocytes. Depending on the physical and chemical characteristics of a substance, the stratum corneum may be crossed transcellularly, meaning via the corneocytes (see Figure). Potential absorption via hair follicles has also been discussed (see Figure). The transfollicular pathway may be especially relevant for particularly hirsute animal skin.
The absorption of substances in and through the skin into the body can be influenced by many different factors (see Table).
Factors which influence dermal absorption
(per Kilo (2017) Hautabsorption. In: Schmitz-Spanke, et al.; Umweltmedizin).
Einflussfaktoren der dermalen Absorption. (nach Kilo (2017) Hautabsorption. In: Schmitz-Spanke et al.; Umweltmedizin)
| Characteristic | Influencing factor | |
| Skin | physiological/ anatomical/physical/ chemical |
age / condition / anatomical location (metabolic) temperature hydration of the stratum corneum |
| Substance | physikalisch/ chemisch |
molecular radius (molekular wight) water or lipid solubility (log KOW) dosage |
| Solvent | physical/ chemical |
viscosiy temperature „penetration enhancers”) |
| Exposure | area time |
| In-vivo : | Measurement in the in-tact organism Studies with test subjects, e.g. intradermal microdialyses, biomonitoring … |
| Ex-vivo: | Measurementoutsideof the organism inphysiologically and metabolically in-tact systems Studies on excised skin, e.g. diffusion-cell studies, microdialysis … |
| In-vitro: | Measurement in artificial systems Studies on artificial cell membranes (e.g. diffusion-cell studies) or in cell cultures … |
| In-silico: | Theoretical determinations of dermal-absorption mechanisms Calculations and computer simulations; e.g. according to diffusion principles, experimental datan |
The gold standard for the evaluation of dermally absorbed substances are in‑vivo human studies. Since animal skin is considerably distinct from human skin in terms of the thickness of the stratum corneum and the number of hair follicles, it is only partially suitable for penetration studies. Since the skins of various species may also vary with respect to their enzymatic properties/activity, human studies (in vitro or ex vivo) are preferred for the evaluation of substances which are intradermally converted to a pharmacologically (“prodrug”) or toxicologically relevant metabolite (Kilo et al. 2016).
funded by the Berufsgenossenschaft Energie Textil Elektro Medienerzeugnisse (BG HM)
Contact person: PD Dr. med. Sonja Kilo
Page contents are currently under revision.
Systemic absorption of organic UV filters from sun-protection products in humans
funded by the Bayerisches Staatsministerium für Gesundheit und Pflege
Contact person: Dr. med. Julia Hiller
Sun is important component of everyday life for humans and provides a wide variety of positive effects – it harbours, however, the risk of short- and long-term damage to the skin via UV radiation. Sun-protection products should protect against these harmful rays, such that their use is propagated to all demographic groups to protect against UV radiation. Due to the broad usage of light-protection products, the question arises as to potential absorption into the human body.
The primary goal of our research is to estimate exposure with respect to transdermal penetration of the most common sun-protection products used for humans in Germany (ethylhexyl salicylate, octocrylene, and avobenzone). As part of an in‑vivo study with volunteers, the internal exposure and excretion of these substances and their metabolites were investigated in healthy subjects after proper use of typical sun-protection products under real-world application conditions. The results of the in‑vivo conditions were compared with those of ex‑vivo methods using additional diffusion-cell tests, thereby enabling conclusions to be made about alternative routes of absorption (e.g. oral ingestion, hand-mouth contamination).
funded by the Berufsgenossenschaft Energie Textil Elektro Medienerzeugnisse (BG ETEM)
Contact person: PD Dr. med. Sonja Kilo (Dr. Gintautas Korinth)
Hydrofluoric acid is the aqueous solution of hydrogen fluoride. Due to its properties, which are clearly distinct from those of other acids, hydrofluoric acid is essential for some industrial branches, such as the petrochemical industry (refinement of mineral oils during fuel manufacture) and the electronics industry. In these industries, the ability of hydrofluoric acid to corrode glass (e.g. dissolve silicates) is employed in the production of circuit boards. Hydrofluoric acid is also required as a starting substance for the production of chemical fluoride compounds. Polytetrafluoroethylene (Gore-Tex®, textile refinement) is one such compound.
From a chemical perspective, hydrofluoric acid is a relatively weak acid. Since is barely dissociates in an aqueous solution and is only present in a slightly hydrated state, the lipophilic hydrofluoric acid can easily penetrate into and through the skin. This characteristic leads to hydrofluoric acid functioning as a contact poison, especially at lower concentrations, which means it can be absorbed into the skin without damaging its surface. In contrast, hydrofluoric acid presents an immediate, superficial, and acid-typical corrosive injury pattern in higher concentrations. The greatest danger posed by hydrofluoric acid to humans is its effect as a systemic calcium scavenger. Calcium is essential for many physiological and biological processes, which can only partially take place, if at all, in cases of insufficient calcium supply. Physiologically, calcium loss causes a shift in other ions (potassium, among others), which leads to an ion imbalance and potentially to cardiac arrest.
Phase I: Development of a human-skin ex-vivo model for the investigation of skin damage by irritating chemical substances using hydrofluoric acid as an example
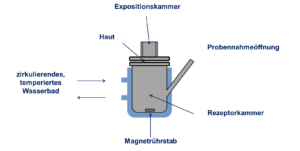
Using the diffusion-cell method established by Franz (1975), excised human skin is examined with respect to the dermal penetration of hydrofluoric acid.
Figure 4: Static diffusion cell per T. J. Franz (Percutaneous absorption: On the relevance of in vitro data. J Invest Dermatol 64: 190–195).
Since diffusion cells can keep excised human skin viable for a limited period of time, the skin can react to irritants to a certain extent. At a certain point in time during hydrofluoric-acid exposure, any skin changes are characterised macroscopically and histologically. Moreover, biochemical examinations for the measurement of early skin damage are performed.
Phase II – Investigation of the efficacy of various recommended skin-cleansing measures (antidotes) for the decontamination of the skin after hydrofluoric-acid exposure with the model developed in Phase I of the study
Due to the systemically toxic and locally destructive consequence of dermal exposure to hydrofluoric acid, even at low concentrations, it is absolutely necessary to test decontamination measures. First-aid recommendations include:
- thoroughly rinsing the skin with water and then massaging in a 2.5% calcium-gluconate gel
- when using Hexafluorine® (Prevor), which was specially developed for hydrofluoric-acid exposure, it is recommended to treat the skin without washing beforehand
- emergency plans recommended polyethylene glycol (PEG) 400 (Lutrol®, BASF) as the primary cleansing agent before treatment with a calcium-gluconate gel
- in some cases, PEG 400 is recommended as the sole antidote for the practice of occupational medicine
In this phase, a skin-cleansing model must be developed based on the model for examination of fluoride penetration after hydrofluoric-acid contamination, as developed in Phase I; moreover, common decontamination strategies used in practice must be compared.
Phase III – Investigation of early decontamination measures after hydrofluoric-acid exposure, of the long-term penetration behaviour of fluoride, of changes to skin temperature and intradermal pH after hydrofluoric-acid exposure, and alternative decontamination strategies
In this phase, the following questions must be examined in more detail:
- efficacy of early decontamination after hydrofluoric-acid exposure
- investigation of the long-term penetration behaviour (over 72 hours) of fluoride after hydrofluoric-acid exposure
- investigation of intradermal pH and skin temperature after hydrofluoric-acid exposure
Phase IV 1 – Investigation of alternative decontamination strategies after careful consideration of intradermal pH after hydrofluoric-acid exposure
In this phase, it must be examined how a shift in pH influences the penetration of fluoride through human skin.
If applicable, various decontamination techniques should be evaluated against the background of all new information and adapted wherever necessary.
Contact person: PD Dr. med. Sonja Kilo
Page contents are currently under revision.
Contact person: PD Dr. med. Sonja Kilo
Page contents are currently under revision.
Contact person: PD Dr. med. Sonja Kilo
Page contents are currently under revision.